electron config for sulfur|Electron Configuration for Sulfur (S) : Manila How to Write the Electron Configuration for Neon. Neon is the tenth element with a . Mustang Gold | Stilvoller Slot von Pragmatic Play mit 25 Gewinnlinien und verschiedenen Bonus-Features | Coole Protagonisten und gute Chancen auf große Gewinne . jackpot €15 000 000. . Dieses Symbol bedeutet Sofortgewinne in Höhe des Einsatzes bis hin zum „Jackpot“. Außerdem gibt es ein besonderes Geldsammelsymbol, sogenannte Collect .
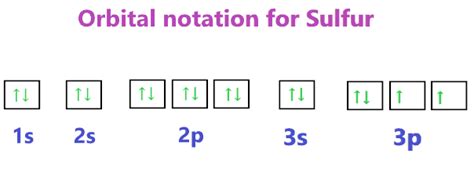
electron config for sulfur,In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons). When we write the configuration we'll put all 16 electrons in orbitals around the nucleus of the Sulfur atom.
How to Write the Electron Configuration for Neon. Neon is the tenth element with a .In order to write the Argon electron configuration we first need to know the .

In order to write the Calcium electron configuration we first need to know the .
Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 .
Therefore the Chlorine electron configuration will be 1s 2 2s 2 2p 6 3s 2 .In writing the electron configuration for nitrogen the first two electrons will go in .In order to write the Mg electron configuration we first need to know the .How to Write the Electron Configuration for Fluorine. Fluorine is the ninth element .
119 rows — Mar 23, 2023 — Electron configuration chart of all Elements is .Nob 18, 2013 — A step-by-step description of how to write the electron configuration for Sulfur (S). In order to write the S electron configuration we first need to know the number of .Abr 8, 2024 — This configuration can be determined through various methods, including the aufbau principle, periodic table organization, Bohr model representation, or orbital diagram visualization. Methods. Aufbau principle. .
Ene 26, 2021 — Ground State Electron Configuration of Sulfur. when we the electron configuration of Sulfur the first two electrons go in the 1s orbital. As 1s only hold two electrons and the next two electrons for sulfur goes to the 2s .Hun 27, 2024 — Find the full electronic configuration and valence electrons of any periodic element using this electron configuration calculator.
Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. Boiling pointThe .
Ago 29, 2022 — The electron configuration of sulfur is 1s2 2s2 2p6 3s2 3p4. A step-by-step description of how to write the electron configuration for Sulfur (S). Explain It To A Child. The most common configuration of electrons for sulfur is .
Electron configuration for sulfur. The history of Sulfur. Periodic table history. Identifiers. List of unique identifiers for Sulfur in various chemical registry databases. Sulfur is a chemical .The ground state electron configuration of ground state gaseous neutral sulfur is [Ne].3s 2.3p 4 and the term symbol is 3 P 2. Schematic electronic configuration of sulfur. . Electron binding energies for sulfur. All values of electron binding energies are given in eV.electron config for sulfurThe arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. . (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, .

Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. Boiling point . But reduce sulfur by giving it a couple of electrons, and its smell is unmistakable. The requirement of sulfur reduction to sulfide has clearly been lost .
Electron Configuration for Sulfur (S) Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs. Boiling point . But reduce sulfur by giving it a couple of electrons, and its smell is unmistakable. The requirement of sulfur reduction to sulfide has clearly been lost .Ago 29, 2022 — What is the sulfur electron configuration? Sulfur is a nonmetal element with an atomic number of 16. This means that it has 16 protons in its nucleus. The sulfur electron configuration lists the different ways that sulfur can arrange its electrons. The most common sulfur electron configuration is 1s2 2s2 2p6 3s2 3p4.May 28, 2024 — Consider sulfur's electron configuration, which was determined in the previous section and is replicated below. 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4. Recall that the energy levels in an electron configuration are the leading red numbers that denote the start of a new energy level / orbital combination. Sulfur has electrons in the first, second, and .The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum number of the .
Dis 28, 2022 — The electron configuration of an atom indicates the number of valence electrons. Valence electrons determine the unique chemistry of each element. Skip to main content . The electron configuration for sulfur is 1s 2 2s 2 2p 6 3 s 2 3p 4 and can be represented using the orbital diagram below. Exercises .
Ene 26, 2021 — Sulfur Electron Configuration: Sulphur or sulfur is a chemical element. It has a chemical symbol S. The atomic number of Sulfur is 16. It is multivalent, abundant, and nonmetallic. Under normal situations, sulfur forms cyclic octatomic molecules that have a chemical formula S 8.Electronic configuration for Sulfur (S) The atomic number of Sulfur is 16. Sulfur belongs to group 16 of the Modern periodic table. In the periodic table, Sulfur is placed in the third period. For the electronic configuration of an element there are three important rules which must be followed: Aufbau Principle Pauli-exclusion Principle Hund's .Ago 17, 2023 — Let's find the ground state electron configuration of Sulfur! A single Sulfur atom has 16 protons and 16 electrons, but how do we know where Sulfur puts its .
electron config for sulfur Electron Configuration for Sulfur (S) Ago 17, 2023 — Let's find the ground state electron configuration of Sulfur! A single Sulfur atom has 16 protons and 16 electrons, but how do we know where Sulfur puts its .For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration (also known a spdf notation) . The element is Sulfur, S. d) 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p .Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.May 28, 2024 — Since all of sulfur's electrons have been placed, this final entry represents sulfur's electron configuration. Example \(\PageIndex{1}\) Write the electron configuration for nitrogen. Solution. In order to write an electron configuration for nitrogen (N), the total number of electrons in an atom of nitrogen must first be determined.Okt 12, 2023 — The electron configuration of Sulfur in terms of the shell or orbit is [2, 8, 6]. The ground-state electron configuration of the Sulfur (S) atom is 1s 2 2s 2 2p 6 3s 2 3p 4. And for the excited state, it is 1s 2 2s 2 2p 6 3s 2 3p 3 3d 1. The shorthand electron configuration for Sulfur is [Ne] 3s 2 3p 4. The electron configuration for the .
Mar 17, 2015 — The 14 electrons will fill up as follows: 1s^(2)2s^(2)2p^(6)3s^(2)3p^(4) Or 2. 8. 6. Here are a couple videos to help with the concept of electron configurations. video from: Noel Pauller video from: Noel PaullerThe same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a noble gas, .Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and 2p subshells .Electron configuration 3s 2 3p 4: Electrons per shell: 2, 8, 6: . They use sulfur as the electron acceptor, and reduce various oxidized sulfur compounds back into sulfide, often into hydrogen sulfide. They can grow on other partially oxidized sulfur compounds (e.g. thiosulfates, thionates, polysulfides, sulfites). .
electron config for sulfur|Electron Configuration for Sulfur (S)
PH0 · Sulfur electron configuration
PH1 · Sulfur Electron Configuration (S) with Orbital Diagram
PH2 · Sulfur Electron Configuration
PH3 · Sulfur (S)
PH4 · Sulfur
PH5 · Electron Configuration for Sulfur (S)
PH6 · Electron Configuration Chart of All Elements (Full Chart)
PH7 · Electron Configuration Calculator
PH8 · Complete Electron Configuration for Sulfur (S, S2